Background of Cobalt:
Cobalt, the element that is just starting to create a lot of buzz, from the auto industry to electronics production, the popularity of cobalt is growing rapidly, chiefly for its use in rechargeable batteries. As global demand for cobalt increases, current sources of cobalt may not be sufficient. This imbalance between supply and demand may open doors for novel methods of recovery and recycling of cobalt.
Centuries ago, before the development of batteries, cobalt had other primary uses in construction, paints, and medicines. Cobalt can be magnetized, has high-temperature strength, hardness, and corrosion resistance. It is these properties that make cobalt such a popular metal to use in alloys with aluminum and nickel, and in stainless steel.
Cobalt deposits are found naturally, with the largest known deposits in the Democratic Republic of the Congo (DRC), Canada, Australia, Zambia, and Brazil. The crustal abundance of cobalt is 26.6 ppm, falling significantly below the relative abundances of nickel, zinc, and copper in the Earth.
With a current global recycling rate for cobalt of approximately 25% for purchased scrap, it continues to pose a significant supply risk. Cobalt forecasts suggest a rapid increase in demand in the coming years, especially with the push for electric vehicles. Recycling of cobalt and cobalt recovery in general are about to become hot topics, and important areas of interest for miners, producers, and primary users of cobalt.

Current Mining Methods for Cobalt Production:
Cobalt is found primarily with mineral deposits of nickel and copper in the Earth. There are many different cobalt ores that are mined, involving many steps of separation and purification. The most well-known and mined ores are: nickel cobalt sulfide, copper cobalt sulfide, copper cobalt oxide, nickel cobalt laterite, and cobalt arsenic ores.
Cobalt arsenides are processed using a roasting stage, followed by a pressure leach. Due to health and environmental issues, almost all mines recovering these cobalt ores have been closed down.
Copper-cobalt (oxides and sulfides) has a more complex series of steps that are followed to purify cobalt. Copper-cobalt ores are primarily found in Zambia and the DRC, where the cobalt is recovered from copper flotation concentrates, using a roast-leach-electrowin (RLE) process. The ore is roasted (for sulfides), followed by a sulfuric acid atmospheric leach, and direct electrowinning of copper. Impurity removal is a key step, as well as cobalt hydroxide precipitation. The final steps involve a re-leach and cobalt electrowinning for cobalt recovery. Newer plants involve copper solvent extraction-electrowinning (SX-EW), instead of direct copper electrowinning, which leads to LME Grade ‘A’ copper. The copper flotation step is inefficient for cobalt, such that there is only a 40% recovery for oxides, and a maximum of 80% for sulfides. The tailings dams have significant amounts of cobalt that could potentially be recovered, in addition to slags that have high cobalt content as well. It is in these low-grade waste streams where cobalt recovery could be optimized by the use of subsequent processing and electrowinning.
Nickel sulfide deposits are processed using a variety of methods to produce both nickel and cobalt products. One very well-known process involves an ammonia leach, otherwise known as the Sherritt-Gordon process, using hydrogen reduction. The modified Sherritt-Gordon process is a pressure oxidation leach. Other methods include a sulfate oxidative leach, a chlorine leach – followed by chloride electrowinning, electro-refining of nickel matte anodes, and electro-refining of impure metal.
Nickel laterites (oxides) are processed using Caron or HPAL hydrometallurgical methods, because these methods allow for the recovery of cobalt, whereas pyrometallurgical processing will not.
A generalized process for cobalt recovery may include the following steps:
Step 1) Impurity Removal
- Impurities are precipitated with “lime” (CaCO3 – limestone, or Ca(OH)2 – milk of lime)
Step 2) Solvent Extraction (SX)
- Removal of copper, zinc, manganese, and cobalt-nickel separation
Step 3) Ion Exchange (IX)
- Removal/polishing of zinc, copper, and nickel
Step 4) Final Purification (Cobalt Recovery)
- Re-leach and electrowinning cobalt
- Can electrowin in tandem with SX and IX, such as SX-emew or IX-emew systems
Cobalt oxide was the main form of cobalt that was desired for many years, as it was used as a blue colourant. Post WWII, demand for cobalt metal skyrocketed because of jet engines and gas turbines. At the same time, demand for nickel metal increased because it played a significant role as a component of stainless steel. This parallel demand for nickel and cobalt led to nickel sulfide deposits being heavily mined for their high nickel and cobalt content. As we move towards a more technology-driven society, pure cobalt metal is more desirable for use in batteries and semiconductors in electronics and electric vehicles (EVs). Roughly 25% of cobalt produced is metallic, primarily serving high-performance aerospace and defence superalloys. Cobalt metal can be produced by electrowinning - an area that we at emew are well-versed in.
Step-by-step, we can look at the different methods used to recover cobalt, of which there are many. Purification and recovery of cobalt can prove to be very difficult. Cobalt and nickel are found together not only in the Earth, but they are also found side-by-side on the periodic table. They have similarities in their aqueous chemistry that make them very difficult to separate. Both cobalt and nickel are divalent, hexahydrated ions in dilute aqueous solutions, although the water exchange rate for cobalt is much higher than that of nickel, so cobalt forms complex ions more easily. Traditional separation methods for cobalt and nickel are based on the selective oxidation and/or precipitation of cobalt from either sulfate or chloride solution. Another common method is solvent extraction by alkylammines. Because of their similarities, the processes used to recover nickel and cobalt are very similar. A generalized flow sheet of the recovery of copper, cobalt, and nickel can be found below, Figure 1.

Fig. 1: Generalized Flow Sheet of Copper/Cobalt/Nickel Recovery (Fisher, 2011)
When designing processes for cobalt recovery, it is important to analyze and overcome some of the technical challenges that lie ahead. In terms of impurities, manganese is the most troublesome impurity for cobalt recovery, specifically from copper-cobalt ores, in which Mn is present in high quantities. Air/SO2 or O2/SO2 mixtures are used to oxidize and precipitate manganese from sulfates, commercially adopted at DRC copper-cobalt plants (Tenke, Fungurume, and Ruashi). The air/SO2 method has the fewest disadvantages, especially at the larger scale.
Many improvements to cobalt processing have been implemented to alleviate some of the more difficult challenges with cobalt recovery; these include, but are not limited to:
- Bio-leaching
- Heap bio-leach
- Bio-leaching for cobaltiferous pyrates
- Cobalt recovery from copper smelter slag
- Crystallization for cobalt sulfates
- Evaporation/crystallization can give a high value if a good grade, but it is dependent on Mn and Mg removal
- Problems with this method include its complexity, potential for gypsum contamination and scaling, and high energy costs
- Benefit: no reagents are required
- High temperature/pressure leaches for cobalt arsenides
- HPAL for nickel laterites
- Favoured over the Caron process or pyrometallurgical methods, HPAL has advanced to use larger autoclaves for higher throughputs
- Hydrogen reduction for briquettes
- Cobalt powder is produced by hydrogen reduction in an autoclave
- Electrowinning is usually preferred to this method, for lower capital and operating costs, and higher metal recovery
- Molecular recognition technology (MRT)
- MRT is similar to IX, but uses a special resin that can target specific molecules
- Can use this method in tandem with electrowinning, such as an MRT-emew system
- Precipitation of cobalt as an intermediate product
- A variety of reagents can be used to obtain different intermediate products
- NaHS (sodium hydrosulfide) or H2S to produce cobalt sulfide
- Ca(OH)2 for low-grade hydroxide/basic sulfate containing gypsum
- Na2CO3, produces high-grade cobalt carbonate
- NaOH, produces high-grade cobalt hydroxide
- MgO (magnesia), produces high-grade cobalt hydroxide
- All of these, with the exception of cobalt sulfide, are excellent feeds for electrowinning cobalt
- The sulfide route is a common choice in the nickel industry because it rejects manganese, however it is not as common for cobalt as the process is complex and the product less saleable
- Magnesia route is specialized, applied to mixed nickel-cobalt hydroxide (MHP) production in Australian laterite projects
- Benefits to the magnesia route:
- Cleaner, lower mass product
- Easy materials
- Hydroxide cake is easily filtered and re-leached
- Some Mn rejection
- MgSO4, Mg can be precipitate with lime
- Problems with the magnesia route
- Large quantities of high-grade MgO
- Imported from USA or Australia
- Reagent is vulnerable to hydration and aging
- CaO in the MgO forms gypsum in the product
- Grades of MgO vary in effectiveness
- Benefits to the magnesia route:
- A variety of reagents can be used to obtain different intermediate products
- Resin-in-pulp (RIP)
- Resin is used to load target metals from a slurry, extracting cobalt from low-concentration, impure aqueous stream – transferring to high-concentration, high-purity solution for cobalt electrowinning
- Cobalt intermediate precipitation and re-leach processes are not required here, and manganese and magnesium are rejected
- Can neutralize the pulp directly with lime
- Strong oxidants for hydroxy-oxides
- Aimed to oxidize and precipitate cobalt preferentially over nickel
- Strong oxidants like ozone, Caro’s acid, or chlorine are used, making it very specialized and requires high safety management
- SX is usually preferred to this method, due to poor cobalt/nickel selectivity
- Whole ore leaching (WOL)
- Applied to copper oxide ores, using additional acid to achieve improved recoveries in comparison to oxide ore flotation and concentrate leach
Variation within the processing steps and methods are based on the type of ore that is extracted, and the form of cobalt desired (ie. cobalt sulfide, or mixed Ni-Co sulfide precipitate (MSP); cobalt hydroxide, or mixed Ni-Co hydroxide (MHP); cobalt carbonate; cobalt oxide or oxy-hydroxide; cobalt sulfate; cobalt cathode; or cobalt powder). Each of these different forms of cobalt, metallic or compound, have different applications and end-uses, which is why there are many different methods for cobalt recovery, from various types of cobalt-containing ores.

End Uses of Cobalt:
Although cobalt was first used over 2000 years ago, new uses for this metal continue to put pressure on supply. Rechargeable batteries and electronics are the areas where cobalt is receiving the most attention, and recycling of cobalt from these devices is about to become a very important area of focus. Cobalt is used in electronics, either in their batteries, integrated circuits, and/or semiconductors. Rechargeable batteries contain cobalt oxide, cobalt hydroxide, or cobalt metal. According to the Cobalt Institute, rechargeable batteries now account for approximately 73% of global cobalt consumption, a massive increase driven by the electric vehicle (EV) sector. Consequently, the European Union (under the Critical Raw Materials Act) and the United States (via the USGS/DOE Critical Minerals lists) continue to classify cobalt as a critical raw material due to its economic importance and high risk of supply disruption. The most commonly used type of battery at present is the lithium-ion battery, where cobalt is found in the cathode. Cobalt is also an important component of nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries. These batteries exist in portable devices, such as phones, laptops, tablets, and cutting tools; in EVs; and in stationary batteries, such as renewable energy power stations and home storage.

When thinking of the sustainable future of our planet, a lot of us think about renewable energy sources, but cobalt also plays an important role in making our current energy sources a little greener. Cobalt acts as a catalyst for desulfurization of natural gas and refined petroleum products. Just one ton of cobalt, when applied as a catalyst mixture, can reduce sulfur oxide (SOx) and nitrogen oxide (NOx) emissions by 25 000 tons and 750 tons, respectively. Cobalt is also used as a catalyst in the production of recyclable plastics.
Older, more traditional uses of cobalt are in alloys and inks and pigments. Cobalt plays a major role in alloys used for aerospace, prosthetics, automobiles, and industrial equipment. Cobalt oxide is characteristic to giving glass, porcelain, ceramics, paints, and inks a very bright blue colour. Modern uses of cobalt extend to healthcare, where cobalt metal isotopes are used for detection of tumours, and for radiotherapy. Cobalt is also used for measuring vitamin B12 absorption and to diagnose B12 deficiency – since cobalt is at the center of the vitamin B12 molecule.
With so many uses of cobalt, ranging from new technologies to established industrial applications, cobalt reserves continue to be depleted. The DRC holds 55% of the world’s known cobalt reserves, and this supply won’t last forever. Many countries have been investing in new methods to supply their metals requirements, including alternatives to traditional mining and refining techniques. Recyclability of cobalt is a very important topic of discussion, as is recovery of cobalt from nickel ores and other metal waste streams.
Maximizing Recovery – Electrowinning Cobalt:
As battery technologies continue to receive unprecedented demand and attention, the need for nickel has scaled even faster than early-decade projections. While 2017 reports anticipated significant growth, BloombergNEF and the IEA now confirm that global primary nickel demand reached 3.35 million tonnes in 2024 and is on track to exceed 4.2 million tonnes by 2030. Despite this record demand, the market is currently in its fourth consecutive year of surplus due to Indonesian expansion. This oversupply is reflected in the price: after peaking at over 25,000 USD/tonne in 2022, the price of nickel has cooled significantly, sitting at 15,450 USD/tonne (~15.45 USD/kg) as of December 24, 2025 - a level notably lower than the 2017 highs of ~20 USD/kg.
One important question is how these future demands will be met by suppliers? Only so much cobalt can be extracted per year at each mine, and there are only so many natural reserves, which are readily being exploited. Environmental sustainability and responsibility are key issues as with any resource development project. This leaves cobalt producers to look at alternative methods for production of cobalt, notably cobalt recycling and cobalt recovery from complex, polymetallic secondary sources. Although cobalt can be a pesky metal to electrowin, it can be one of the best ways to recover cobalt from low-concentration solutions. Cobalt can also be recovered by emew systems in conjunction with SX, IX, or MRT. This recovery and recyclability of cobalt is very important to the future of cobalt in our society, in the batteries that power our devices and our cars.
The production of large quantities of cobalt cathode has posed many challenges to those who electrowin this metal. The solution must be extensively purified, and cobalt is highly stressed when it plates onto the cathode. This stress increases with deposition time, and eventually this deposit will just peel itself off the blank. Fortunately, because cobalt metal is highly desired, many professionals have put their energy into determining better techniques for electrowinning cobalt.
Traditional cobalt electrowinning uses full sheet cathodes and undivided cells, but mines in Zambia and the DRC use blanks that don’t have edge strips, so the cobalt grows around the edges and cannot peel off the blank. There is extensive manual effort required using this method, in order to strip the cobalt from the blanks, which in turn can damage them. Another downside to this method is the hydrogen gas evolution from the acid cell; if the cobalt extraction or bite (ΔCo) is above 10 gpl, there is too much H2 gas produced, and the current efficiency decreases to intolerable levels. Low ΔCo also implies that high flow rates are needed. An SX-emew system has the advantage of being able to take a larger cobalt bite due to improved mass transfer and gas handling, leading to enhanced cobalt recovery, and less acid required for the strip; the solution circulating back into the IX or SX feed would have lower cobalt and higher acid concentrations, in comparison to traditional cobalt SX-EW. A further advantage of emew for cobalt electrowinning is the closed nature of the cell which eliminates acid and cobalt mist from the work environment.
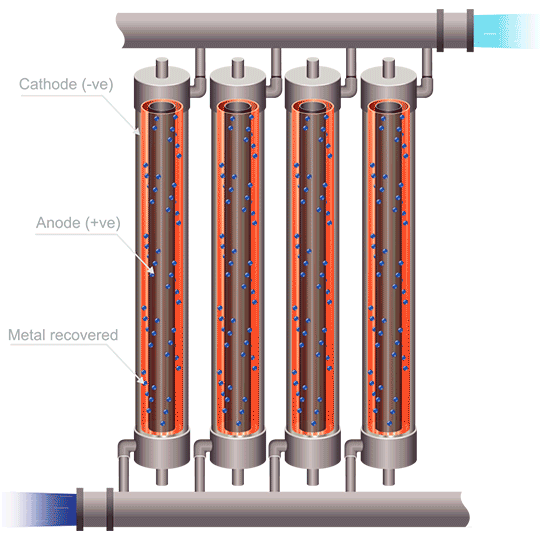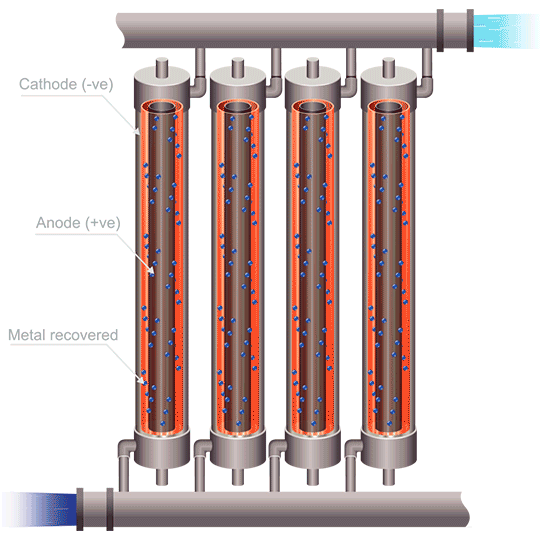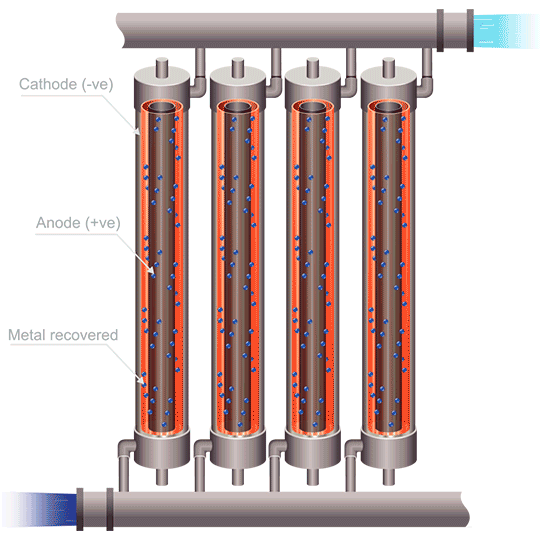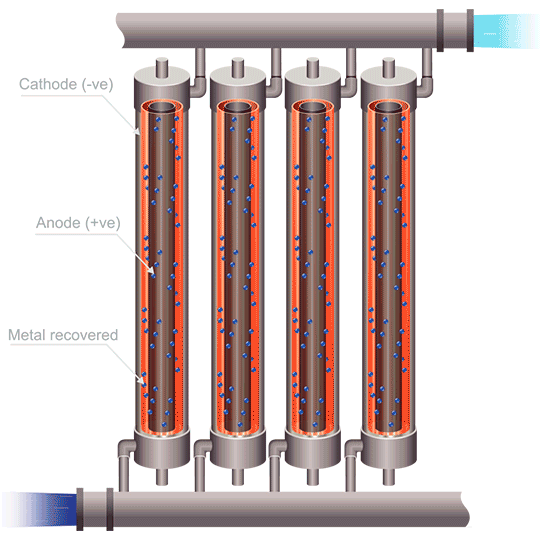
Division of cells can be carried out either by the use of anode bags, or cathode bags. For cobalt electrowinning, anode bags are commonly used; otherwise the full sheet of cobalt could peel off of the cathode and destroy the bag. Anode bags allow for recirculation – and improved flow, of the catholyte; pH can be controlled, giving better current efficiency. Mist acid can be captured, which is another very important benefit of electrowinning cobalt using anode bags. The downsides to this method are that solid anode products can form (MnO2, PbO2, PbSO4, and Co3+ oxides), as well as the danger of one failed or ripped anode bag compromising the entire metal production in a cell.
The use of emew electrowinning technology provides many advantages over conventional cobalt electrowinning. The ability to produce cobalt powder by this method avoids any problems of the highly stressed cobalt metal peeling from the blanks. The high mass transfer of an emew cell also allows for higher current efficiency and use of higher current densities, leading to increased extraction of cobalt. Cobalt electrowinning can produce cobalt with purities exceeding 99%. Implementing cobalt electrowinning in order to maximize cobalt recovery can be an effective method for obtaining pure cobalt metal, and opens the door to better methods to recycle cobalt in the future.

Cobalt Recovery, what the Future Holds:
Cobalt is a metal that is contributing to a greener planet, as a catalyst for desulfurization of natural gas and refined petroleum products, and as a key component in rechargeable batteries, in both mobile and stationary devices. It’s important to analyze the life cycle of cobalt, how cobalt recovery can be maximized, and how to better promote and implement cobalt recycling. It seems counterproductive to extract cobalt in such an environmentally destructive way, only to integrate it into technologies and products that are considered “environmentally friendly”, or “sustainable”.

With such a promising outlook for so many sustainable technologies utilizing cobalt that impact our daily lives, it’s time for cobalt buyers, and suppliers, to think about how cobalt can be recovered in a more sustainable manner, and how innovative cobalt recycling methods can be implemented,
Recovery of cobalt and cobalt recycling are areas that need more focus, with attention to technologies such as electrowinning, reliable for extracting cobalt from low concentration waste streams, and scrap. Although cobalt electrowinning can be challenging, continued research and development of this technique for cobalt recovery is making it more practical even for small-scale operations. Maximizing recovery of cobalt from current cobalt extraction processes, as well as end of life devices and products containing cobalt, will close the loop on the life cycle of cobalt. It is this sort of closed loop process, and full circle thinking, that will make it possible for cobalt to remain a fundamental part of our environmentally friendly and sustainable future.
References:
Cobaltinstitute.org. (2017). Cobalt Institute - formerly the Cobalt Development Institute (CDI). [online] Available at: https://www.cobaltinstitute.org [Accessed 22 Sep. 2017].
Fisher, K. (2011). Cobalt Processing Developments. Bateman Engineering Projects. Available at: https://www.saimm.co.za/Conferences/BM2011/237-Fisher.pdf [Accessed 18 Sep. 2017].
Ft.com. (2017). Funds switch on to battery commodities. [online] Available at: https://www.ft.com/content/ced7d86a-2fe9-11e7-9555-23ef563ecf9a [Accessed 18 Sep. 2017].
Infomine.com. (2017). Cobalt Prices and Cobalt Price Charts - InvestmentMine. [online] Available at: http://www.infomine.com/investment/metal-prices/cobalt/ [Accessed 18 Sep. 2017].
Mulaudzi, N. and Kotze, M. (2013). Direct cobalt electrowinning as an alternative to intermediate cobalt mixed hydroxide product. Mintek. Available at: http://www.basemetals.org/WhiteRiver2013/209-Mulaudzi.pdf [Accessed 18 Sep. 2017].
Periodictable.com. (2017). Abundance in Earth's Crust for all the elements in the Periodic Table. [online] Available at: http://periodictable.com/Properties/A/CrustAbundance.v.html [Accessed 22 Sep. 2017].
Rsc.org. (2017). Cobalt - Element information, properties and uses | Periodic Table. [online] Available at: http://www.rsc.org/periodic-table/element/27/cobalt [Accessed 22 Sep. 2017].






